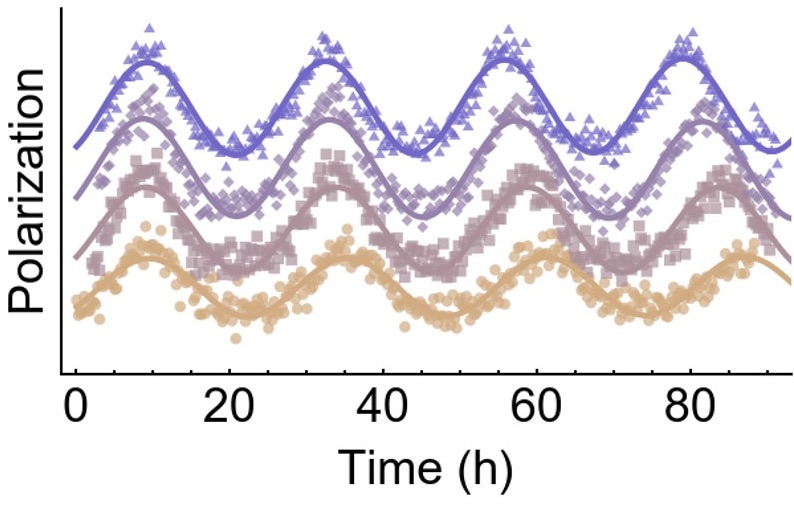
Photosynthetic cyanobacteria (you may know them as blue-green algae) have a remarkably simple circadian clock. Though these bacteria have daily self-sustaining rhythms in their physiology similar to our own, the molecular machinery behind the cyanobacterial clock can be reduced to a mixture of three proteins called KaiA, KaiB, and KaiC. Even when taken outside of a living cell, these proteins continue to function together to produce robust rhythms in a test tube.
Our group and others have been working to understand what happens during the clock cycle. The structure and dynamics of the Kai proteins themselves must encode the 24-hour length of the day-night cycle, but how this happens is not clear. We are using a combination of mutagenesis approaches together with molecular dynamics simulations, in collaboration with Aaron Dinner's group, to understand this structure-function relationship. We recently found evidence that KaiA may act to stimulate KaiC by destabilizing the interface between KaiC subunits, allowing it to act as a nucleotide exchange factor. We are also developing techniques to allow an algorithm to learn the properties of the reaction network in the clock by fitting large amounts of data.
A fundamental property of biological clocks is their ability to respond sensitively to changes in the environment while nevertheless keeping the clock speed nearly unchanged. Importantly, the period of the clock is compensated against changes in temperature over a wide range. This is true even for the Kai protein system, surprising since many biochemical reactions accelerate dramatically when temperature increases. We are using this system to try to understand the physical basis of temperature compensation.
Another design issue is that all biochemical oscillators must consume free energy and resist molecular noise to avoid running down and ultimately stopping. The problem is particularly acute in bacteria where there may be stringent constraints on the amount of protein synthesis and energy consumption that are allocated to a clock. We are interested in both how natural systems deal with this limitations, and which biochemical architectures perform best in energy-limited or stochastic regimes.