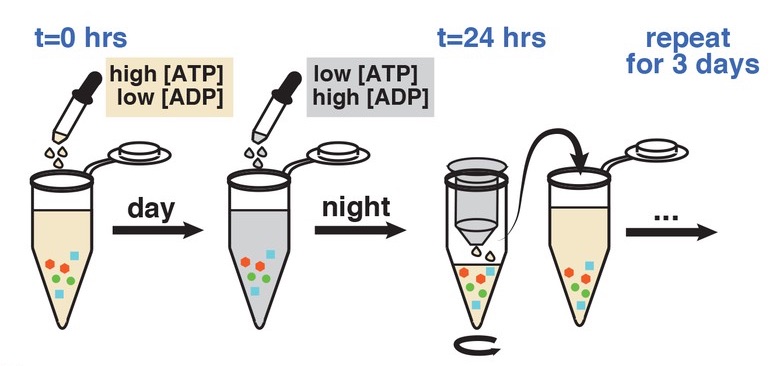
An emerging concept in the study of biological clocks is their intimate coupling to metabolism. For example, our livers have clocks that are attuned to the rhythms of our food intake. Along with other groups, we've found that this link between metabolism and circadian rhythms is even more intimate in the cyanobacterial circadian clock, the simplest biological clock known. We found that no direct light-sensing mechanism is needed in cyanobacteria, and that the Kai proteins that form the circadian oscillator sense key energy metabolites. Manipulating the metabolic state of a cell can override the effects of light and dark. Similarly, creating pulses of metabolites in a test tube makes it possible to simulate the effect of different light-dark cycles on the clock.
However, the role of the clock is not as simple as acting as a sensor of metabolism. The clock also creates rhythms in metabolism, for example, inhibiting glycogen storage in the early part of the day. This accumulation of energy reserves in turn affects the metabolic impact of nightfall. Thus a more accurate description is that the clock is embedded in a metabolic feedback loop. We are working to understand the consequences of this system architecture, especially in more realistic environments where light levels may fluctuate unpredictably during the day.
All of these discoveries suggest that the impacts of clock dysfunction may be closely connected to metabolic dysregulation. We are pursuing both single-cell microscopy and mass spectrometry based approaches to understand the impact of clock-environment misalignment.